Hypoventilation dramatically increases carbonic acid concentration and involves – Hypoventilation, a condition characterized by inadequate breathing, dramatically increases carbonic acid concentration, leading to a cascade of physiological responses. This intricate relationship between hypoventilation and carbonic acid concentration warrants exploration, as it holds significant implications for blood pH and overall bodily functions.
When ventilation is compromised, the body’s ability to eliminate carbon dioxide (CO2) is impaired. As CO2 accumulates, it reacts with water to form carbonic acid (H2CO3), a weak acid that can alter blood pH. This increase in carbonic acid concentration leads to a decrease in pH, a condition known as respiratory acidosis.
Hypoventilation and Carbonic Acid Concentration
Hypoventilation, or inadequate breathing, leads to a buildup of carbon dioxide (CO 2) in the blood. This increase in CO 2concentration drives the formation of carbonic acid (H 2CO 3) through the following chemical reaction:
CO2+ H 2O ⇌ H 2CO 3
The accumulation of carbonic acid contributes to the development of respiratory acidosis, a condition characterized by a decrease in blood pH.
Effects of Elevated Carbonic Acid on pH, Hypoventilation dramatically increases carbonic acid concentration and involves
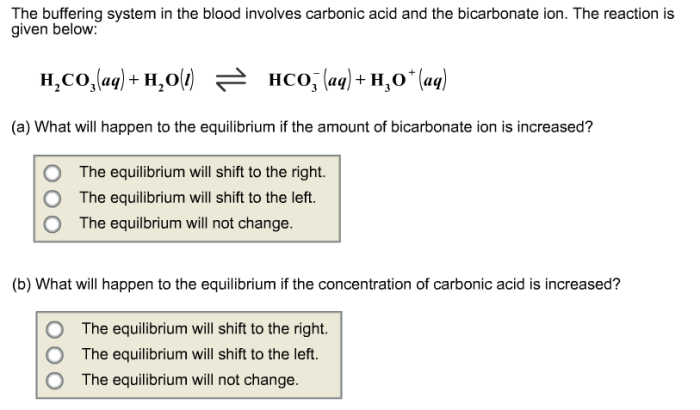
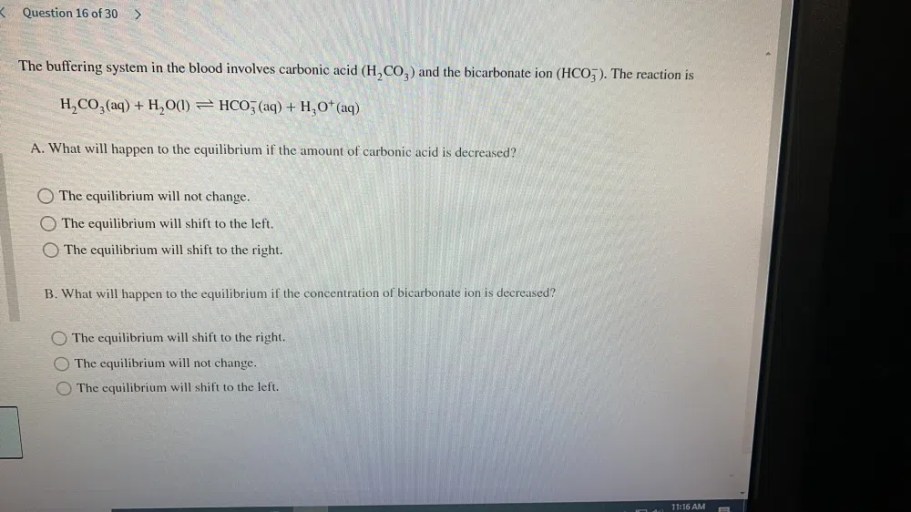
Elevated carbonic acid concentration lowers blood pH by increasing the concentration of hydrogen ions (H +). This decrease in pH is caused by the dissociation of carbonic acid into hydrogen ions and bicarbonate ions (HCO 3–):
H2CO 3⇌ H ++ HCO 3–
The decrease in pH can have detrimental effects on bodily functions, including enzyme activity, protein structure, and cellular metabolism.
Respiratory Compensation Mechanisms
The body employs respiratory mechanisms to compensate for hypoventilation and restore normal pH levels. These mechanisms include:
- Increased respiratory rate: This increases the elimination of CO 2and reduces the concentration of carbonic acid.
- Increased tidal volume: This increases the amount of air exchanged during each breath, further promoting CO 2elimination.
However, these compensatory mechanisms have limitations and may not be sufficient to fully correct the pH imbalance caused by severe hypoventilation.
Clinical Implications of Hypoventilation
Hypoventilation can occur in various clinical conditions, including:
- Chronic obstructive pulmonary disease (COPD)
- Neuromuscular disorders
- Drug overdose
Signs and symptoms of hypoventilation include:
- Shortness of breath
- Confusion
- Cyanosis
Untreated hypoventilation can lead to severe complications, such as respiratory failure and death.
Clarifying Questions: Hypoventilation Dramatically Increases Carbonic Acid Concentration And Involves
What are the primary mechanisms responsible for the increase in carbonic acid concentration during hypoventilation?
The primary mechanisms include impaired CO2 elimination due to reduced ventilation and the subsequent reaction of CO2 with water to form H2CO3.
How does elevated carbonic acid concentration affect blood pH?
Elevated carbonic acid concentration lowers blood pH, leading to respiratory acidosis.
What are the potential consequences of untreated hypoventilation?
Untreated hypoventilation can lead to severe complications, including respiratory failure, organ damage, and even death.