Understanding conceptual components of the enthalpy of solution – Embark on a scientific odyssey to unravel the intricacies of enthalpy of solution, a fundamental concept that governs the energetic interplay in chemical systems. This discourse will delve into its conceptual components, illuminating the interplay between solutes, solvents, and their intricate interactions.
Understanding enthalpy of solution not only enriches our comprehension of chemical reactions but also unlocks its practical applications in industrial processes. Join us as we unveil the secrets of this captivating concept, unlocking a deeper understanding of the molecular world.
Enthalpy of Solution Concepts

Enthalpy of solution refers to the change in enthalpy that occurs when a solute dissolves in a solvent. This process can be either exothermic, releasing heat, or endothermic, absorbing heat. The enthalpy change associated with the dissolution process is influenced by various factors, including the nature of the solute and solvent, their interactions, and the environmental conditions.
Components of Enthalpy of Solution: Understanding Conceptual Components Of The Enthalpy Of Solution
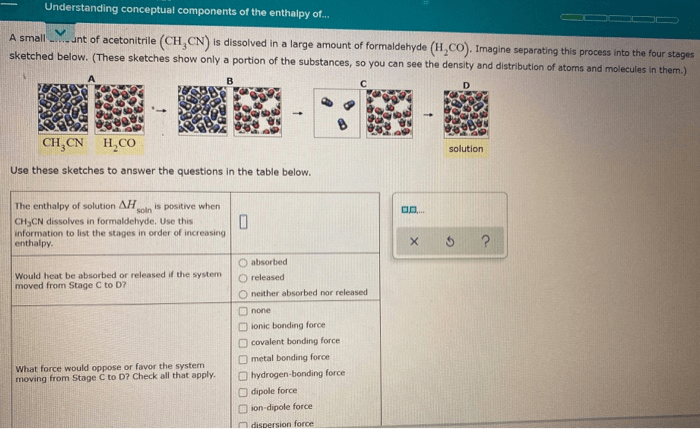
The enthalpy of solution comprises several components that contribute to the overall enthalpy change:
Solute-Solvent Interactions, Understanding conceptual components of the enthalpy of solution
These interactions involve the formation of new bonds between solute particles and solvent molecules. The strength and type of these bonds determine whether the process is exothermic or endothermic.
Solvent-Solvent Interactions
The dissolution process disrupts the solvent-solvent interactions, which can either release or absorb heat depending on the nature of the solvent.
Solute-Solute Interactions
In concentrated solutions, solute particles can interact with each other, leading to changes in enthalpy. These interactions can be attractive or repulsive, affecting the overall enthalpy of solution.
Factors Affecting Enthalpy of Solution
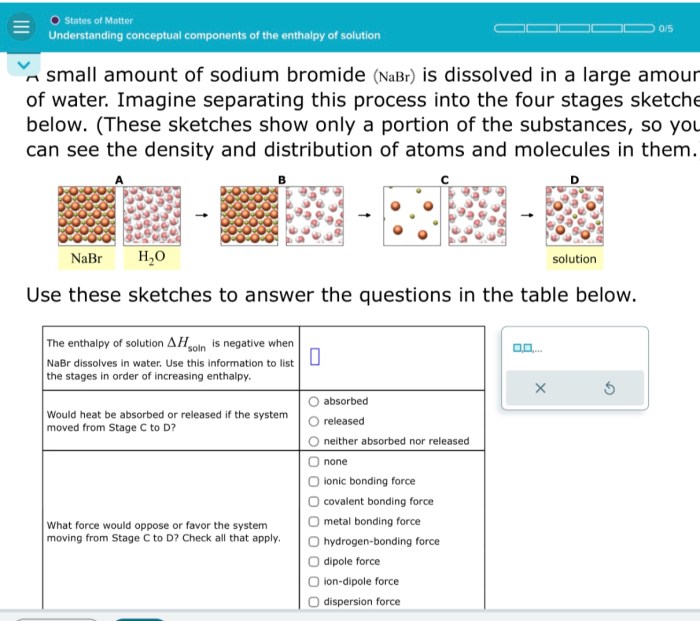
The enthalpy of solution is influenced by several factors:
- Temperature:Temperature affects the kinetic energy of the molecules, influencing the strength and nature of solute-solvent interactions.
- Pressure:Pressure can affect the solubility of gases in liquids, impacting the enthalpy of solution.
- Solute Concentration:The concentration of the solute can influence the extent of solute-solute interactions, affecting the enthalpy of solution.
- Solvent Properties:The polarity, viscosity, and dielectric constant of the solvent can influence the strength of solute-solvent interactions.
Applications of Enthalpy of Solution

Enthalpy of solution has various applications:
- Chemical Reactions:Enthalpy of solution is used to determine the heat released or absorbed during chemical reactions in solution.
- Industrial Processes:Enthalpy of solution is crucial in designing and optimizing industrial processes, such as crystallization and solvent extraction.
- Solubility Predictions:Enthalpy of solution data can be used to predict the solubility of substances in different solvents.
Helpful Answers
What is enthalpy of solution?
Enthalpy of solution is the change in enthalpy that accompanies the dissolution of a solute in a solvent.
What are the different types of enthalpy of solution processes?
Enthalpy of solution processes can be either exothermic (heat is released) or endothermic (heat is absorbed).
What factors affect the enthalpy of solution?
The enthalpy of solution is affected by factors such as the nature of the solute and solvent, temperature, and pressure.
What are the applications of enthalpy of solution?
Enthalpy of solution has applications in various fields, including chemistry, engineering, and materials science.