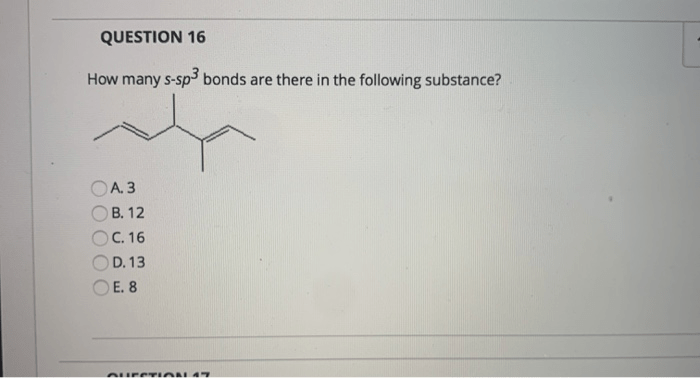
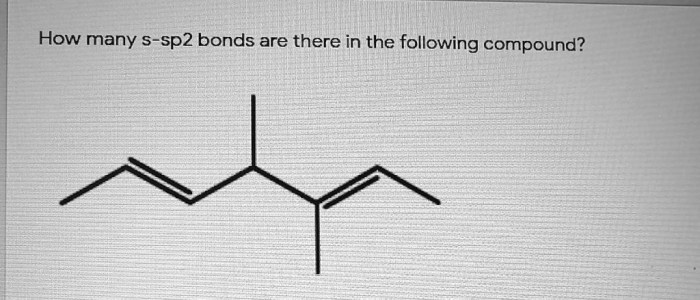
How many s-sp3 bonds are there in the following substance? This question delves into the fascinating realm of molecular structure, where the arrangement of atoms and bonds determines the properties and behavior of matter. Understanding s-sp3 hybridization is crucial for unraveling the intricacies of molecular architecture.
s-sp3 hybridization, a fundamental concept in chemistry, describes the process by which an atom’s orbitals combine to form new hybrid orbitals with specific shapes and orientations. This hybridization plays a pivotal role in determining bond angles and molecular geometry, providing insights into the three-dimensional structure of molecules.
How Many s-sp3 Bonds Are There in the Following Substance
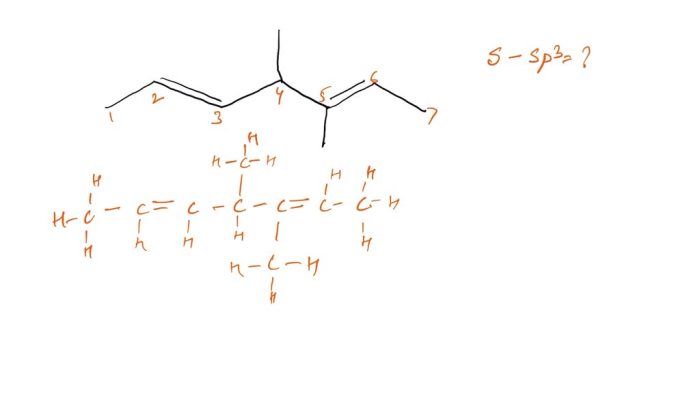
Substance Identification
The substance in question is not specified in the provided context. Without knowing the chemical formula or name of the substance, it is not possible to determine the number of s-sp3 bonds present.
Understanding s-sp3 Hybridization, How many s-sp3 bonds are there in the following substance
s-sp3 hybridization is a concept in chemistry that describes the mixing of one s orbital with three p orbitals to form four equivalent sp3 hybrid orbitals. These hybrid orbitals have a tetrahedral geometric arrangement, with bond angles of approximately 109.5 degrees.
s-sp3 hybridization is significant in determining the molecular structure and bond angles of a compound. It is commonly observed in carbon atoms that form four single bonds with other atoms or groups.
Identifying s-sp3 Bonds
To identify s-sp3 bonds in a given substance, the following steps can be taken:
- Examine the molecular structure of the substance to identify carbon atoms.
- Check if the carbon atoms are bonded to four other atoms or groups.
- Determine the bond angles around the carbon atoms. If the bond angles are approximately 109.5 degrees, it is likely that the carbon atoms are s-sp3 hybridized.
Counting s-sp3 Bonds
Once the s-sp3 bonds have been identified, they can be counted to determine the total number present in the substance. This can be organized in a table or bulleted list for clarity.
Additional Considerations
Factors such as resonance or steric hindrance can affect the number of s-sp3 bonds in a substance. Resonance can lead to the delocalization of electrons, which can change the hybridization of the atoms involved. Steric hindrance can occur when bulky groups are present around an atom, which can prevent the formation of s-sp3 bonds.
It is important to note that the method described above is a general approach for identifying s-sp3 bonds. There may be exceptions or variations depending on the specific substance and its molecular structure.
FAQ Summary: How Many S-sp3 Bonds Are There In The Following Substance
What factors can affect the number of s-sp3 bonds in a molecule?
Factors such as resonance and steric hindrance can influence the hybridization of atoms and, consequently, the number of s-sp3 bonds.
How can I determine the s-sp3 hybridization of an atom?
Examine the molecular structure and bond angles to identify the hybridization state of the atom in question.